Esophageal Varices
Recommendations
1. Introduction Esophageal Varices
Esophageal varices are Porto-systemic collaterals — i.e., vascular channels that link the portal venous and the systemic venous circulation. They form as a consequence of portal hypertension (a progressive complication of cirrhosis), preferentially in the sub mucosa of the lower esophagus. Rupture and bleeding from esophageal varices are major complications of portal hypertension and are associated with a high mortality rate. Variceal bleeding accounts for 10–30% of all cases of upper gastrointestinal bleeding.
1.1 WGO Cascades – a resource-sensitive approach
A gold standard approach is feasible for regions and countries where the full scale of diagnostic tests and medical treatment options are available for the management of esophageal varices. However, throughout much of the world, such resources are not available. With Diagnostic and Treatment Cascades the WGO Guidelines provide a resource sensitive approach.
Cascade: a hierarchical set of alternative diagnostic, therapeutic and management options to deal with risk and disease - ranked by resources available.1.2 Epidemiology
Although varices may form in any location along the tubular gastrointestinal tract, they most often appear in the distal few centimeters of the esophagus. Approximately 50% of patients with cirrhosis develop gastroesophageal varices. Gastric varices are present in 5–33% of patients with portal hypertension.
The frequency of esophageal varices varies from 30% to 70% in patients with cirrhosis (Table 1), and 9–36% of patients have what are known as “high-risk” varices. Esophageal varices develop in patients with cirrhosis at an annual rate of 5–8%, but the varices are large enough to pose a risk of bleeding in only 1–2% of cases. Approximately 4–30% of patients with small varices will develop large varices each year and will therefore be at risk of bleeding.
Table 1 Epidemiology of esophageal varices and correlation with liver disease
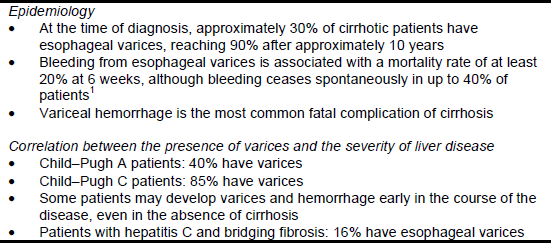
The presence of gastroesophageal varices correlates with the severity of liver disease. The severity of cirrhosis can be scored using the Child–Pugh classification system (Table 2).
Table 2 Child-Pugh classification of the severity of cirrhosis
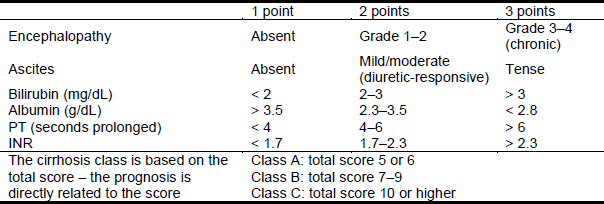
INR, international normalized ratio; PT, prothrombin time.
1.3 Natural history
A cirrhosis patient who does not have varices has not yet developed portal hypertension, or his or her portal pressure is not yet high enough for varices to develop. As portal pressure increases, the patient may progress to having small varices. With time, and as the hyperdynamic circulation increases, blood flow through the varices will increase, thus raising the tension in the wall. Variceal hemorrhage resulting from rupture occurs when the expanding force exceeds the maximal wall tension. If there is no modification in the tension of the wall, there will be a high risk of recurrence.
Table 3 – Prognosis in patients with esophageal varices
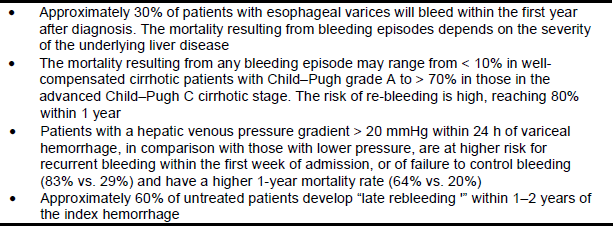
Figure 1 – Natural history of varices and hemorrhage in patients with cirrhosis2
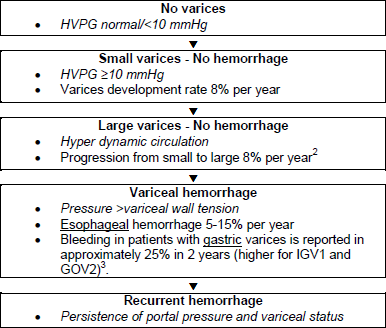
HVPG = hepatic venous pressure gradient; IGV = isolated gastric varices in absence of esophageal varices located in gastric fundus; GOV2 = gastroesophageal varices extending along greater curvature toward gastric fundus
1.4 Risk factors
An international normalized ratio (INR) score > 1.5, a portal vein diameter of > 13 mm, and thrombocytopenia have been found to be predictive of the likelihood of varices being present in cirrhotics. If none, one, two, or all three of these conditions are met, then < 10%, 20–50%, 40–60%, and > 90% of the patients are estimated to have varices, respectively. The presence of one or more of these conditions represents an indication for endoscopy to search for varices and carry out primary prophylaxis against bleeding in cirrhotic patients (Table 4).
Table 4 – Risk factors for esophageal varices and hemorrhage
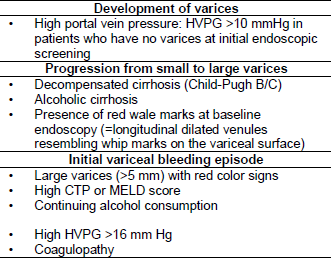
2. Diagnosis and Differential Diagnosis
Esophagogastroduodenoscopy is the gold standard for the diagnosis of esophageal varices. If the gold standard is not available, other possible diagnostic steps would be Doppler ultrasonography of the blood circulation (not endoscopic ultrasonography). Although this is a poor second choice, it can certainly demonstrate the presence of varices. Further alternatives include radiography/barium swallow of the esophagus and stomach, and portal vein angiography and manometry.
It is important to assess the location (esophagus or stomach) and size of the varices, signs of imminent, first acute, or recurrent bleeding, and (if applicable) to consider the cause and severity of liver disease.
Table 5 - Guideline for diagnosing esophageal varices
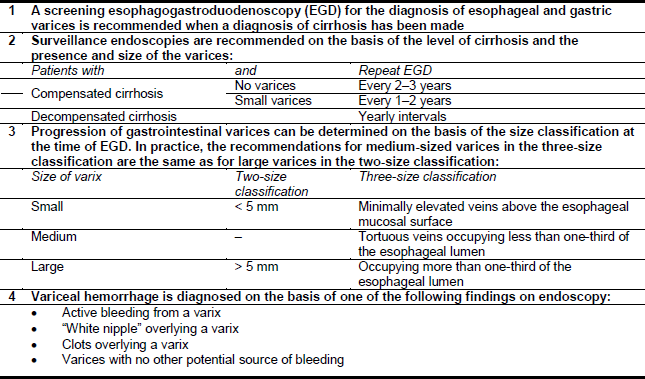
2.1 Differential diagnosis of esophageal varices/hemorrhage
The differential diagnosis for variceal hemorrhage includes all etiologies of (upper) gastrointestinal bleeding. Peptic ulcers are also more frequent in cirrhotics.
Table 6 - Differential diagnosis of esophageal varices/hemorrhage
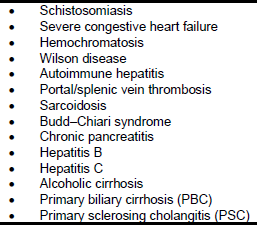
Note: all of these lead to the development of esophageal varices as a result of portal hypertension.
2.2 Example from Africa — esophageal varices caused by schistosomiasis
Schistosomiasis is the most common cause of varices in the setting of developing countries — in Egypt or the Sudan, for example. In absolute numbers, it may be a more common cause than liver cirrhosis. In the Sudan, there are villages in which over 30% of the population have varices. Their liver function is well maintained. They rarely decompensate and do not develop hepatocellular carcinoma (HCC). Bleeding from varices is the main cause of death in these patients. If the varices are eradicated, the patients can survive more than 25 years.
2.3 Other considerations
Table 7 - Considerations in the diagnosis, prevention, and management of esophageal varices and variceal hemorrhage
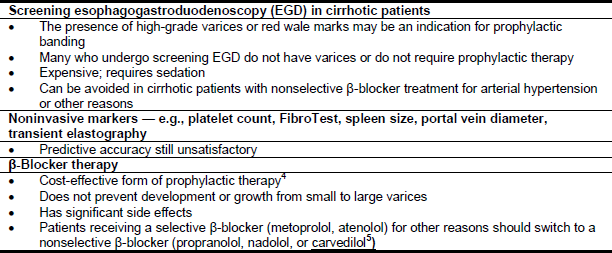
3. Management of Varices and Hemorrhage
The following treatment options are available in the management of esophageal varices and hemorrhage (Tables 8 and 9). Although they are effective in stopping bleeding, none of these measures, with the exception of endoscopic therapy, has been shown to affect mortality.
Table 8 – Pharmacological therapy
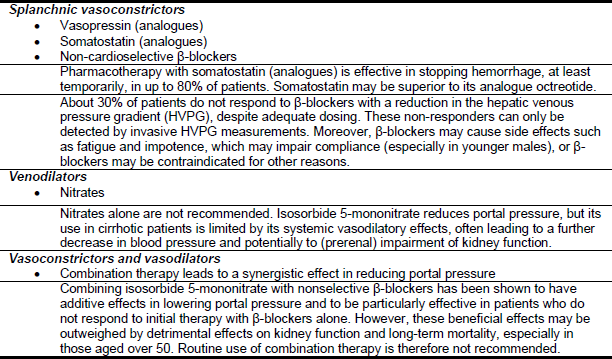
- The use of vasoactive drugs may be safe and effective whenever endoscopic therapy is not promptly available and is associated with less adverse events than emergency sclerotherapy.6
Table 9 – Endoscopic therapy

- Endoscopic sclerotherapy and variceal band ligation are effective in stopping bleeding in up to 90% of patients. EVL is more effective than endoscopic variceal sclerotherapy (EVS) with greater control of hemorrhage, lower rebleeding, and lower adverse events but without differences in mortality.7, 8 However, endoscopic band ligation may be more difficult to apply than sclerotherapy in patients with severe active bleeding.
- A transjugular intrahepatic portosystemic shunt (TIPS) is a good alternative when endoscopic treatment and pharmacotherapy fail.
- The use of balloon tamponade is decreasing, as there is a high risk of rebleeding after deflation and a risk of major complications. Nevertheless, balloon tamponade is effective in most cases in stopping hemorrhage at least temporarily, and it can be used in regions of the world where EGD and TIPS are not readily available. It can help stabilize the patient in order to gain time and access to EGD and/or TIPS later.
- Combined endoscopic and pharmacologic treatment is shown to achieve better control of acute bleeding than endoscopic treatment alone.9
3.1 Clinical practice
The approach in patients with cirrhosis and various stages of varices/hemorrhage is shown in the following figures.
Figure 2 - Patients with cirrhosis but no varices. EGD, esophago-gastroduodenoscopy
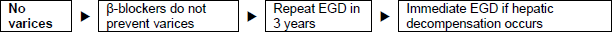
Figure 3 - Patients with cirrhosis and small varices, but no hemorrhage.
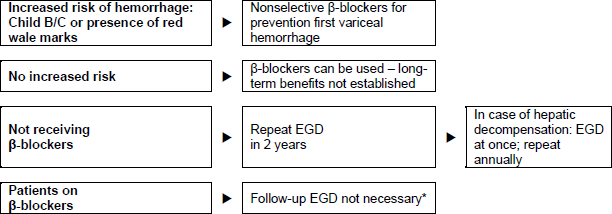
*Because many patients do not respond to β-blocker treatment or bleeding prophylaxis, it is recommended that EGD be repeated after 2 years (as for those not receiving β-blockers).
Figure 4 - Patients with cirrhosis and medium or large varices, but no hemorrhage. EVL, endoscopic variceal ligation.
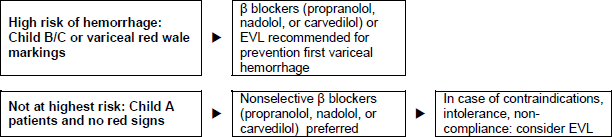
- Non-cardioselective β-blockers (propranolol, nadolol, or carvedilol), starting at a low dosage, if necessary increasing the dose step by step until a reduction in the resting heart rate of 25%, but not lower than 55 beats/min, is reached.
- In comparison with β-blockers, endoscopic variceal ligation was found to reduce bleeding episodes and severe adverse events significantly, but it had no effect on the mortality rate.
Figure 5 – Patients with cirrhosis and acute variceal hemorrhage.
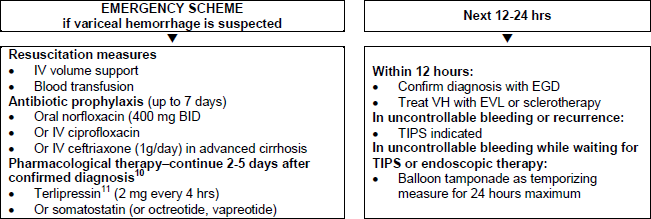
BID, bis in die/twice a day; EGD, esophagogastroduodenoscopy; EVL, endoscopic variceal ligation; IV, intravenous; TIPS, transjugular intrahepatic portosystemic shunt; VH, variceal hemorrhage.
Terlipressin is currently available in much of Europe, India, Australia, and the UAE, but not in the United States or Canada.
- Acute variceal hemorrhage is often associated with bacterial infection due to gut translocation and motility disturbances. Prophylactic antibiotic therapy has been shown to reduce bacterial infections, variceal rebleeding12, and increase the survival rate13.
- In acute or massive variceal bleeding, tracheal intubation can be extremely helpful to avoid bronchial aspiration of blood.
- In patients with variceal hemorrhage in the gastric fundus: endoscopic variceal obliteration using tissue adhesives (such as cyanoacrylate) is preferred; the second choice is EVL.
- TIPS should be considered in uncontrollable fundovariceal bleeding or recurrence despite combined pharmacological and endoscopic therapy.
- Emergency sclerotherapy is not better than pharmacological therapy for acute variceal bleeding in cirrhosis.
- Terlipressin reduces failure to control bleeding and mortality,14 and should be the first choice for pharmacological therapy when available. Where terlipressin is not available, somatostatin, octreotide, and vapreotide could be used.
- Treating esophageal bleeding with somatostatin analogues does not appear to reduce deaths, but may lessen the need for blood transfusions.
Figure 6 – Patients with cirrhosis who have recovered from acute variceal hemorrhage.
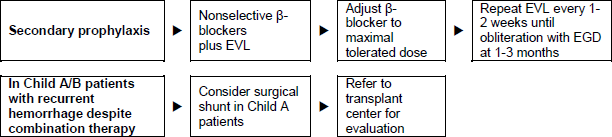
- Long-term endoscopic control and banding or sclerotherapy of recurrent varices every 3–6 months (in many places in the developing world, only sclerotherapy will be available). If endoscopic band ligation is not available or contraindicated, non cardioselective β-blockers (propranolol, nadolol, or carvedilol) starting at a low dosage and if necessary increasing the dosage step by step until a reduction in the resting heart rate by 25%, but not lower than 55 beats/min, is achieved.
- In younger patients with less advanced cirrhosis (Child–Pugh A), the addition of isosorbide 5-mononitrate (starting at 2 × 20 mg per day and increasing to 2 × 40 mg per day) may be considered if sclerotherapy or pharmacotherapy fail. TIPS should be considered, especially in candidates for liver transplantation. In selected cases (patients with well-preserved liver function, stable liver disease), a calibrated H graft or a distal splenorenal shunt (Warren shunt) may be considered.
- Portosystemic shunts are associated with lower rates of variceal rebleeding in comparison with sclerotherapy/banding, but they increase the incidence of hepatic encephalopathy15
- Liver transplantation should always be considered if the patient has Child–Pugh grades B or C.
Recommendations for first-line management of cirrhotic patients at each stage in the natural history of varices (Fig. 7)
Figure 7 – Recommendations for first-line management.
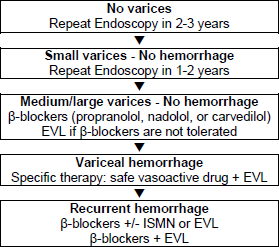
EVL, endoscopic variceal ligation; ISMN, isosorbide 5-mononitrate.
3.2 Cascade for treatment
A cascade is a hierarchical set of diagnostic or therapeutic techniques for the same disease, ranked by the resources available.
As outlined above, several therapeutic options are effective in most clinical situations involving acute variceal hemorrhage, as well as in secondary and primary prophylaxis against it. The optimal therapy in an individual setting very much depends on the relative ease of local availability of these methods and techniques. This is likely to vary widely in different parts of the world.
If endoscopy is not readily available, one has to resort to pharmacotherapy in any case of suspected variceal bleeding — e.g., in patients with hematemesis and signs of cirrhosis. Similarly, pharmacological therapy might be administered in circumstances such as primary prophylaxis in a cirrhotic patient with signs of portal hypertension (splenomegaly, thrombocytopenia) and/or impaired liver function, and as secondary prophylaxis in a cirrhotic patient with a history of upper gastrointestinal bleeding. If pharmacotherapy is also not available and variceal bleeding is suspected, one must resort to general resuscitation measures and transport the patient as soon a possible to an institution where the necessary diagnostic/therapeutic means are available; balloon tamponade could be extremely helpful in such a situation.
Figure 8 – Cascade for the treatment of acute esophageal variceal hemorrhage.
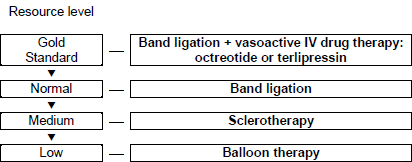
IV, intravenous.
Note: The combination of band ligation and sclerotherapy is not routinely used except when the bleeding is too extensive for a vessel to be identified for banding. In such cases, sclerotherapy can be carried out in order to control the bleeding and clear the field sufficiently for banding to be done afterward.
Caution: There are many conditions that can lead to esophageal varices. There are also many treatment options, depending on the resources available. For a resourcesensitive approach to treatment in Africa, for example, Fedail (2002) can be consulted.
3.3 Example from Africa — esophageal varices and schistosomiasis
Table 10 - Treatment of esophageal varices caused by schistosomiasis

Note: therapy with vasoactive drugs is unrealistic in most developing countries. In the Sudan, for example, 1 mg terlipressin (Glypressin) costs the equivalent of 25% of the salary of a house physician and about the same as a year’s salary for a government employee.
Recommendation Grading
Overview
Title
Esophageal Varices
Authoring Organization
World Gastroenterology Organisation
Publication Month/Year
January 1, 2014
Last Updated Month/Year
May 26, 2023
Supplemental Implementation Tools
Document Type
Guideline
Country of Publication
US
Inclusion Criteria
Male, Female, Adult, Older adult
Health Care Settings
Ambulatory
Intended Users
Nurse, nurse practitioner, physician, physician assistant
Scope
Diagnosis, Assessment and screening, Treatment, Management
Diseases/Conditions (MeSH)
D004932 - Esophageal and Gastric Varices, D004935 - Esophageal Diseases, D004934 - Esophageal Cyst
Keywords
esophageal, Esophageal cancer, Esophageal Varices